OncoExTra® test
Turn unknowns into answers with all-encompassing insights for advanced solid tumors.
Fill in the blanks left by fixed-panel tests.
There are actionable variants that may be missed by fixed-panel tests. The OncoExTra test utilizes whole-exome and whole-transcriptome sequencing to deliver more answers—all in a single test.1*
Uncover more variants. Find more useful targets.
- Ultra-comprehensive genomic profiling across nearly 20K genes in both tumor DNA+RNA, ensuring that all possible protein-coding genes are evaluated for genomic variants up front1
- Broad and deep sequencing coverage accurately detects DNA+RNA variants and rare fusions1
- Patient-matched tumor-normal sequencing provides gold standard TMB calculation2 and allows for differentiation between germline and somatic variants
- Add on single IHC stains and/or preselected tumor-specific panels for even more biomarker detail†
Make meaningful
decisions with
fewer uncertainties
The OncoExTra test may be right for
your patient whose cancer is:




of Patients Who Receive
the OncoExTra® test
have $0 In Out-of-
Pocket Costs3
of Patients Have a
Financial Responsibility
of <$1003
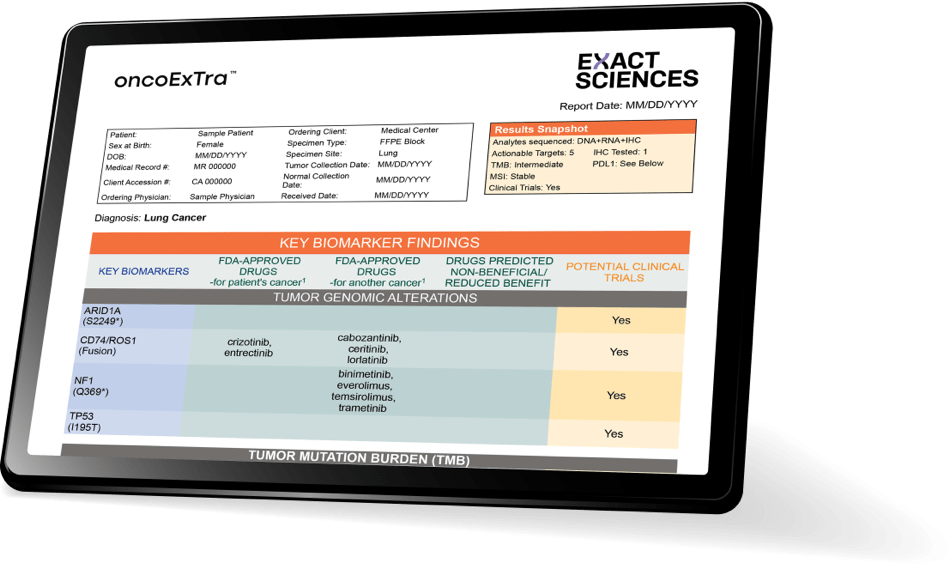
The OncoExTra report:
uncovering what matters most
The easy-to-interpret report highlights the most actionable insights first for timely, shared decision-making:
- Variants and fusions associated with FDA-approved therapies deliver a comprehensive genomic picture‡
- Immuno-oncology signatures (TMB/MSI) support individualized therapy selection
- Clinical trial options to help inform next steps
- Optional IHC panels provide an additional level of detail
Fax your order
* Whole-transcriptome with select variants reported in New York State
† IHC testing not currently available in New York State
‡The OncoExTra test was developed, and the performance characteristics validated by Genomic Health, Inc., a wholly-owned subsidiary of Exact Sciences
Corporation following College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA) regulations. The OncoExTra test is performed at the Genomic Health Phoenix clinical laboratory. Exact Sciences clinical laboratories are accredited by CAP, certified under CLIA regulations, and qualified to perform high-complexity clinical laboratory testing. This test has not been cleared or approved by the US Food and Drug Administration
IHC, immunohistochemistry; MSI, microsatellite instability; TMB, tumor mutational burden.
-
References
